1 Introduction
White LEDs are more and more widely used because they have many advantages that traditional light sources do not have, such as long life, power saving, dimming control, vibration resistance, low voltage driving, and no environmental pollution. ]. At present, the most mature white LEDs use blue LEDs to excite yellow light phosphors to produce white light. In addition, violet or near-ultraviolet LEDs can be used to excite red, green and blue phosphors to produce white light [2-7]. The YAG phosphor suitable for blue light excitation is a relatively mature phosphor. The first commercial white LED is obtained by Japan Nichia Corporation in blue chip and YAG package. However, the color rendering index is not high, strictly speaking, it does not meet the lighting standards. In recent years, there have been many reports on nitride phosphors [8-13]. These phosphors are more difficult to synthesize under high temperature and pressure. In addition, phosphors of the silicate series which are easier to synthesize have been developed [14, 15]. However, there are not many related reports at present.
For further research. Ho Seong Jang et al. [13] reported a Sr3SiO5:Ce3 +, Li+ yellow phosphor. A white LED can be obtained by exciting the phosphor with a 405 nm or 460 nm LED chip. The efficiency of the white LED obtained by using it and the blue chip package is 31. 7 lm /W, Ra = 81, Tc = 6 857 K, and the color coordinate is (x, y) = (0. 308 6, 0. 316 7). On this basis, a small amount of Ba was used to replace Sr in Sr3SiO5. It was found that the emission peak red shifted with the increase of Ba doping content, from 541. 12nm red to 553. 75 nm. In addition, Joung Kyu Park et al. [14] reported that the partial shift of Sr with Ba in Sr3SiO5:Eu can red shift the emission spectrum. Based on this, the effect of replacing Sr with different alkaline earth metals was studied. In addition, the effect of doping different amounts of Eu on the emission intensity in the (SrBa)3SiO5 matrix was also investigated.
2 experiment
Such phosphors are synthesized by a high temperature solid phase method. The reagents used in the experiment were SrCO3(AR), Li2CO3(AR), MgO(AR), CaCO3(AR), BaCO3(AR), SiO2(AR), Eu2O3 (99.99%), CeO2(AR). The experimental design components are: A: (SrBa)3SiO5:0. 024Ce3 +, 0. 024Li+; B: Sr2. 73M0. 2SiO5:0. 07Eu2 +(M = Ba,Mg, Ca) ; C:(SrBa)3SiO5: xEu2 +. The above raw materials were accurately weighed according to the stoichiometric ratio, thoroughly mixed, and placed in a high-temperature furnace in a reducing atmosphere for 1 h at 1 360 °C. The reducing atmosphere is H2 (10%) / N2. The obtained sinter is ground into a powder by an agate mortar, and after sieving, it is subjected to a process such as washing and removing impurities to obtain a finished product. The excitation and emission spectra of the obtained sample were measured by a Fluorolog 23 fluorescence spectrometer.
3 Results and discussion
3. 1 Sample A Series
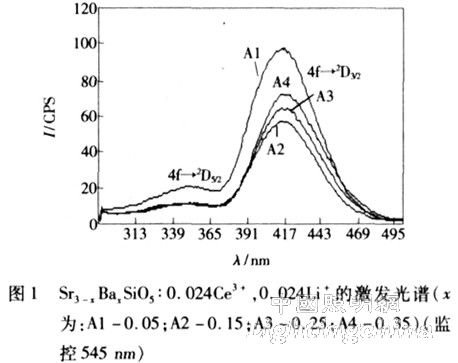
Sample A series is a silicate activated with Ce3+. A small amount of Ba ions was doped on the basis of Sr3SiO5:Ce3 +, Li+ to study the effect of a small amount of Ba ion instead of Sr ions on the spectral characteristics of the sample. Its molecular formula can be written as Sr3 - xBaxSiO5:0. 024Ce3 +, 0. 024Li+ (the contents of x are: A1 -0. 05; A2 - 0. 15; A3 - 0. 25; A4 - 0. 35). The excitation spectra of the four samples are shown in Figure 1, with a monitoring wavelength of 545 nm. From their excitation spectra, it can be seen that sample A has two excitation peaks, which are around 351 and 418 nm, respectively. These two peaks belong to the 4f→2D5/2 (351 nm) and 4f→2D3/2 (418 nm) transitions, respectively. Among them, the peak of 351 nm is lower, and the peak of 418 nm is higher. The full width at half maximum of these two peaks is relatively wide. This may be due to the fact that both the 2D5/2 and 2D3/2 bands of the Ce3+ ions in the crystal are relatively wide at normal temperature. In addition, the 4f energy level containing two sub-levels of 2F7/2 and 2F5/2 may also be one of the reasons. The main excitation peaks of the four samples were: A1 (416 nm), A2 (418 nm), A3 (418 nm), and A4 (418 nm). In general, the incorporation of different amounts of Ba ions did not significantly affect the excitation spectrum of the sample.
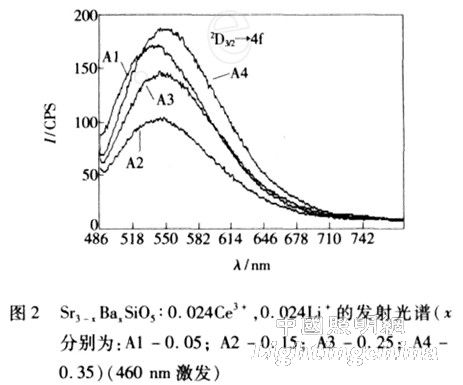
The emission spectra of the four samples are shown in Figure 2 with an excitation wavelength of 460 nm. It can be seen from Fig. 2 that the emission of the four samples is a yellow-green broadband emission with a peak value of about 540-555 nm. This is obtained by the 2D3/2→4f (2F7/2 and 2F5/2) energy level transitions. The emission spectrum has a wide bandwidth, which is advantageous for packaging white LEDs. The emission spectra of the four samples were obtained by Gaussian fitting of the appropriate range of spectral data. The emission peaks of the four samples were: A1 (541. 1 nm), A2 (545. 5 nm), A3 (550. 5 nm). ), A4 (553. 8 nm). The full width at half maximum of the four samples were: A1 (79. 1 nm), A2 (77. 6 nm), A3 (80. 4 nm), and A4 (89. 3 nm). It can be seen that as the content of Ba ions increases, the peak wavelength of the emission spectrum appears red-shifted, from 540 nm red to 550 nm, and the bandwidth also increases. Therefore, by changing the content of Ba ions, the peak wavelength of the emission spectrum can be adjusted, and the color coordinates and color rendering index of the white LED can be adjusted.
3. 2 sample B series
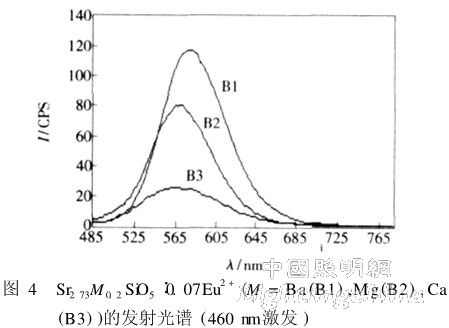
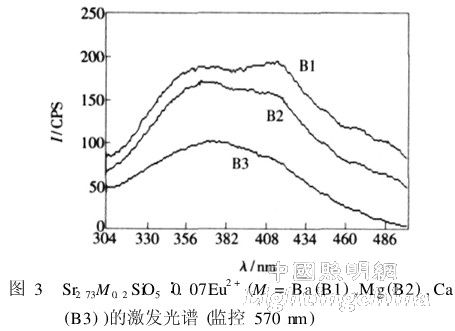
Sample B series is a silicate activated with Eu2+. Substituting Sr with a small amount of other alkaline earth metal elements in the Sr3SiO5 matrix gave different samples. The molecular formula of the sample B series can be written as Sr2. 73M0. 2SiO5:0. 07Eu2 + (M = Ba (B1), Mg(B2), Ca(B3)). The excitation spectra of the three samples are shown in Figure 3, with a monitoring wavelength of 570 nm. The excitation spectra of the three samples are relatively wide, with strong excitation from 340 to 450 nm. Ideal for white LEDs in near-ultraviolet and blue LED chip packages. There are two excitation peaks for sample B1 and sample B2, which are about 367 and 415 nm, respectively, and the peak value of sample B3 at 415 nm is already weak and almost invisible. In addition, sample B1 has an inconspicuous peak at around 470 nm. The peak value of sample B1 at 415 nm is higher than the peak at 367 nm, while the peak value of sample B2 at 415 nm is lower than the peak at 367 nm. In addition, sample B3 has substantially no excitation above 480 nm, and the effect is only equivalent to 1/10 of the peak at 370 nm. Sample B1 also has a partial excitation at 480 nm, and the effect is about half of the peak. It can be seen that in the Sr2. 73M0. 2SiO5:0. 07Eu2 + series, the effect of M is better.
The emission spectra of the three samples are shown in Figure 4 with an excitation wavelength of 460 nm. As can be seen from the figure, all three samples emit a broad spectrum of orange-yellow light. The peak of the emission peak of the sample B1 is 583.1 nm, and the full width at half maximum is 62. 4 nm; the peak wavelength of the emission of the sample B2 is 571. 2 nm, and the full width at half maximum is 62. 0 6 nm。 The peak wavelength of the sample B3 is 571. 5 nm, the full width at half maximum is 75. 6 nm. It can be seen that the emission peak wavelengths of B2 and B3 are almost the same, and the emission peak wavelength of B1 is obviously moving toward the red wave direction. The emission half-peaks of the three samples were similar in full width.